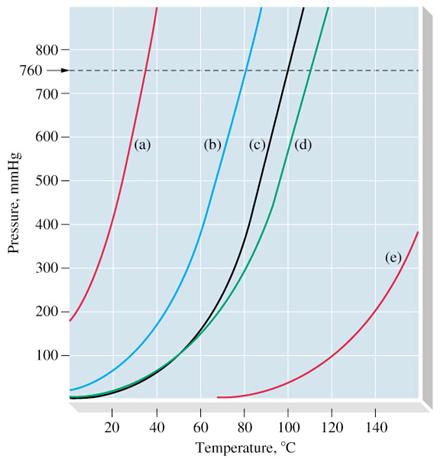
Figure 1. Typical vapour pressure-temperature curves (a) Diethyl ether, (b) Benzene, (c) Water, (d) Toluene, (e) Aniline. 760 mmHg is the standard atmospheric pressure.
Distillation
Distillation is the most common method used to separate and purify liquids. The process consists of heating a liquid to its boiling point, conducting the vapours to a cooling device where they are allowed to condense, and collecting the condensate.
General Principles
The temperature dependent equilibrium vapor pressure: If a liquid is kept in a sealed container, some molecules escape from the liquid's surface into the space above it. In equilibrium the number of molecules evaporating from the liquid is equal to the number of molecules condensating to the liquid. The molecules in the vapour also strike the walls of the container and exert a pressure, defined as the vapour pressure of the liquid. If the temperature of the liquid is raised, a greater number of molecules evaporate until equilibrium is once again established; the vapour pressure of the liquid increases with increasing temperature. Figure 1 shows a typical vapour pressure-temperature curves.

l. Pure elements or compounds
The Boiling Point (Kelvin or Celsius): it is the theperature at which the vapor pressure above the liquid is equals the pressure of its surroundings. A liquid in a vacuum environment has a lower boiling point than when the liquid is at atmospheric pressure. A liquid in a high pressure environment has a higher boiling point than when the liquid is at atmospheric pressure (cf. Figure 1). The boiling point of water is 100 °C (212 °F) at standard pressure. On top of Mount Everest the pressure is about 260 mbar (26.39 kPa) so the boiling point of water is 69 °C. (156.2 °F).
The normal boiling point (Kelvin or Celsius) (also called the
atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special
case in which the vapor pressure of the liquid equals the defined atmospheric
pressure at sea level, 1 atmosphere. At that temperature, the vapor pressure of
the liquid becomes sufficient to overcome atmospheric pressure and lift the
liquid to form bubbles inside the bulk of the liquid. The standard boiling point
is defined by IUPAC (1982) as the temperature at which boiling occurs under a pressure
of 1 bar (100 kPa).
The vapour pressure of a liquid rises exponentially as the temperature increases, until the boiling point is reached (Figure 1).
Liquids may change to a vapor at temperatures below their boiling points through the process of evaporation. Evaporation is a surface phenomenon in which molecules located near the vapor/liquid surface escape into the vapor phase. On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid.
The boiling point of a liquid is also an indicator of the strength of weak attractive forces between the liquid's molecules.
The heat of vaporization, DHvap, is the amount of energy (Joule) required to convert or vaporize a saturated liquid (i.e., a liquid at its boiling point) into a vapor.
A saturated liquid contains as much thermal energy as it can without boiling (or conversely a saturated vapor contains as little thermal energy as it can without condensing).
Saturation temperature means boiling point.
Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapor phase. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased so is saturation temperature.
If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased.
Similarly, a liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure is decreased.
The normal boiling point of water is precisely 99.97 degrees Celsius at a pressure of 1 atm (i.e., 101.325 kPa). Until 1982 this was also the standard boiling point of water, but the IUPAC now recommends a standard pressure of 1 bar (100 kPa). At this slightly reduced pressure, the standard boiling point of water is 99.61 degrees Celsius.
A thermometer placed in the vapour of a boiling pure liquid will record its boiling point. The pressure must be recorded when determining a boiling point.
2. Ideal Mixture of Liquids
If a mixture of two miscible liquids with different boiling points is heated to boiling, the vapour will not have the same composition as the liquid; it will be richer in the more volatile component. Consider Figure 2, which depicts the behaviour of mixtures of A and B, two miscible volatile liquids with boiling points TA and TB, respectively. The lower of the two solid curves represents the boiling points of mixtures of A and B. The upper curve represents the composition of the vapour which is in equilibrium with the liquid as its boiling point. At 100% A or 100% B the curves meet, because boiling pure A (at TA) can have only pure A vapour in equilibrium with it; the same applies to pure B (at TB).
If a mixture of A and B with composition C1 is heated, it will boil at TC1. Reading horizontally on the graph, one sees that the vapour at TC1 will have the composition given by C2. This means that if mixture C1 were placed in a distillation apparatus and heated to its boiling point, the vapour (and therefore the first drop of liquid to be condensed) would have the composition C2; it would be much richer in A, the more volatile of the two components, than was the original liquid.
As the distillation proceeded, A would be selectively removed from the liquid. The composition of the liquid would change gradually from C1 to 100% B. The boiling point of the liquid would gradually rise from TC1 to TB; at the same time, the composition of the distillate would gradually change from C2 (rich in A) to 100% B. Thus, in a simple distillation of a mixture, the first material to distill (sometimes called the first cuts or first fractions) will be rich in the most volatile or lowerboiling components and the last material (last cuts or fractions) will be rich in the less volatile or higherboiling components.
This behaviour is true for "ideal liquids" with very similar, or the same intermolecular interactions (A..A, B..B, and A..B: similar cohesive and adhesive forces), and is approximated by many organic mixtures.
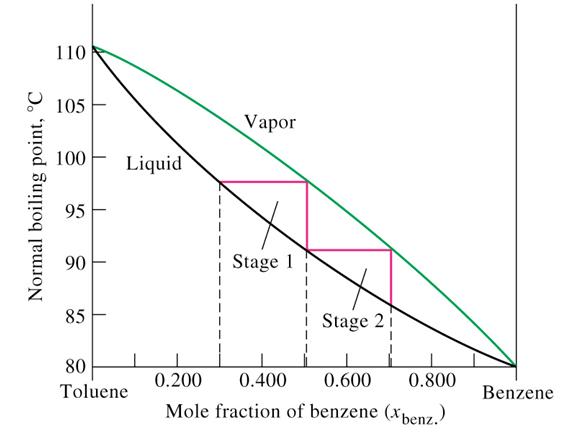
3. Liquids that Form Azeotropes
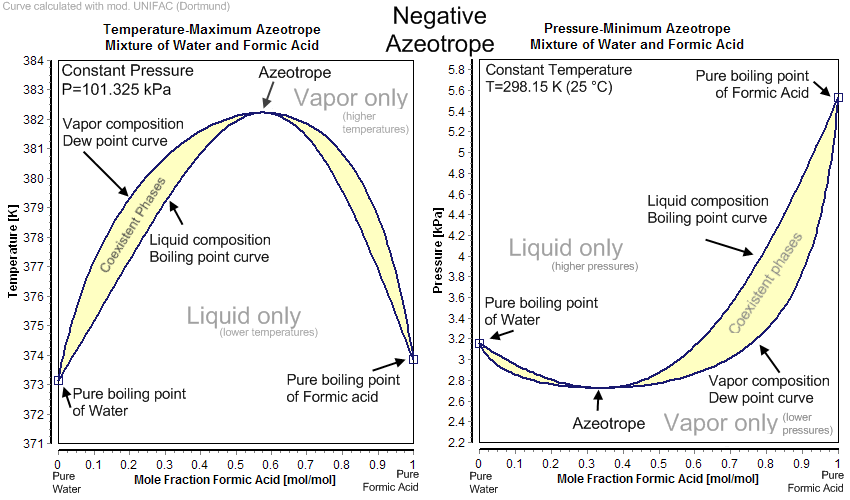
Some liquid mixtures, instead of forming ideal solutions with liquidvapour composition diagrams typified by Figure 2, form either minimum or maximum boiling mixtures because of very different adhesive and cohesive forces. Typical composition diagrams for such mixtures are shown in Figure 3.
Any mixture with a composition exactly equal to that of aseotropic will distill at a single constant temperature, just as if it were a pure liquid. Ethanol and water form a constant boiling mixture (95.6% ethanol, 4.4% water) with a minimum boiling point of 78.2o C (lower than that of pure ethanol, 78.3o C, or pure water, 100o C). Formic acid and water also form a constant boiling mixture (22.5% formic acid, 77.5% water) with a maximum boiling point of 107.1 o C (higher than that of formic acid, 100.8
Water and formic acid solution with a composition to the left of azeotrope in
upper part of Figure 3 can be
separated to pure water and azeotropic mixture, but pure formic acid cannot be
obtained from such a mixture via distillation. Conversely, mixtures with a
composition to the right of azeotrope in Figure 3 can be separated to pure
formic acid and azeotropic mixture, but can never furnish pure water by distillation.
Figure 3: Examples for negative and positive azeotropes.
Fractional Distillation
See also in wiki
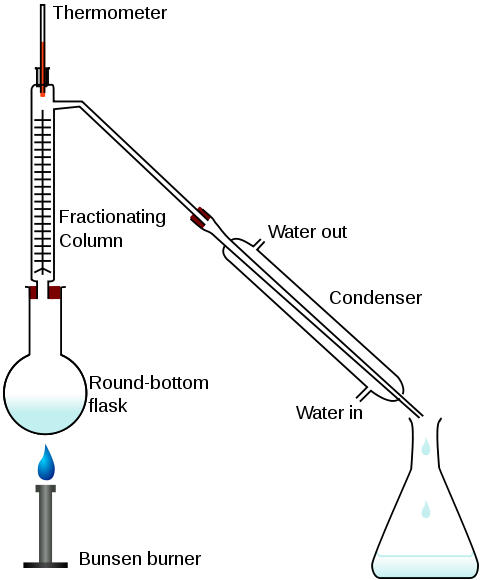
Figure 4: Fractional distillation apparatus using a Liebig condenser. A conical
flask is used as a receiving flask. Here the distillation head and fractionating
column are combined in one piece. [Laurence M. Harwood, Christopher J. Moody.
Experimental organic chemistry: Principles and Practice (Illustrated edition
ed.). pp. 145-147. ISBN 978-0632020171.]
As an example, consider the distillation of a mixture of water and ethanol.
Ethanol boils at 78.4 °C while water boils at 100 °C. So, by gently heating the
mixture, the most volatile component will concentrate to a greater degree in the
vapor leaving the liquid. Some mixtures form azeotropes, where the mixture boils
at a lower temperature than either component. In this example, a mixture of 96%
ethanol and 4% water boils at 78.2 °C, being more volatile than pure ethanol.
For this reason, ethanol cannot be completely purified by direct fractional
distillation of ethanol-water mixtures.
The apparatus is assembled as in the diagram (Figure 4). The mixture is put into
the round bottomed flask along with a few anti-bumping granules (or a Teflon
coated magnetic stirrer bar if using magnetic stirring), and the fractionating
column is fitted into the top. As the mixture boils, vapor rises up the column.
The vapor condenses on the glass platforms, known as trays, inside the column,
and runs back down into the liquid below, refluxing distillate. The column is
heated from the bottom. The efficiency in terms of the amount of heating and
time required to get fractionation can be improved by insulating the outside of
the column in an insulator such as wool, aluminium foil or preferably a vacuum
jacket. The hottest tray is at the bottom and the coolest is at the top. At
steady state conditions, the vapor and liquid on each tray are at equilibrium.
Only the most volatile of the vapors stays in gaseous form all the way to the
top. The vapor at the top of the column, then passes into the condenser, which
cools it down until it liquefies. The separation is more pure with the addition
of more trays (to a practical limitation of heat, flow, etc.) The condensate
that was initially very close to the azeotrope composition becomes gradually
richer in water. The process continues until all the ethanol boils out of the
mixture. This point can be recognized by the sharp rise in temperature shown on
the thermometer
The current laboratory excercise
Experimental Notes and Warnings
1. Before inserting a thermometer or glass tube into a stopper or rubber tube:
Do not attempt to push or pull glass tubing or thermometers
from rubber tubing, corks or stoppers which have become hardened. Cut the
rubber or cork from the glass.
2. When starting the water flow through the condenser do not turn the tap on full blast! A gentle flow will suffice.
3. DO NOT allow the distillation to proceed to complete dryness - this is VERY DANGEROUS. Always leave 1-3 mL of liquid in the distillation "pot".
Part A. Separation of a Binary Mixture by Simple Distillation at Atmospheric Pressure
PROCEDURE
Use the apparatus shown in Figure 5 (use your 100 mL graduated cylinder to collect the distillate, placing the mouth of the cylinder as far as possible into the receiver-adaptor) to distill a mixture of water, acetic acid and a coloring agent. Ensure that the top of the thermometer bulb is positioned midway in the opening to the condenser. Use a Bunsen burner to heat the flask to which has been added a few pieces of pumice stone. Be sure that the apparatus is not "closed" to atmosphere; otherwise pressure will build up and possibly cause an explosion. Control the heat during the distillation so that the distillate drips slowly and steadily into the receiver (about 1 drop per second). Use a graduated cylinder to collect the distillate.
Fill 90 ml liquid into the still pot. Record the temperature every 5 ml as the distillation proceeds until
70 ml of distillate are collected. (See below for how to write this up in your
copybook.)
Please note: This experimental setup is special in that
it uses a graduated cylinder to collect the distillate. This is not how distillations are normally carried out - one normally uses a roundbottom flask to collect with, so that loss of vapours is minimized
as shown in Figure 5 (8: Distillate/receiving flask).
The granules that prevent "bumping" (sudden boyling burst out) due to superheating,
are made from inert material with small pores and provide sites where bubbles can form, thus inducing even boiling. If, during a distillation, the temperature should drop below the boiling point of the liquid in the flask, liquid will fill the pores and the boiling aid will no longer be effective. In this event, the liquid can be cooled and a fresh chip cautiously added. The new chip should not be added when the liquid is at or near the boiling point, as this may initiate violent boiling.
Recording of Results
Construct a table in your notebook to record the temperature at 5 ml of distilled volume.
Plot the boiling point vs. volume distilled.

i) Be certain the hole is large enough to accommodate the glass.
ii) Lubricate the glass and the rubber with glycerol or stopcock
grease.
iii) Protect your hands by holding the stopper and the glass in towels.
Hold the stopper by your fingers NOT IN THE PALM OF YOUR HAND.
iv) Grasp the glass close to the end that is to fit into the stopper and
twist with an even pressure. 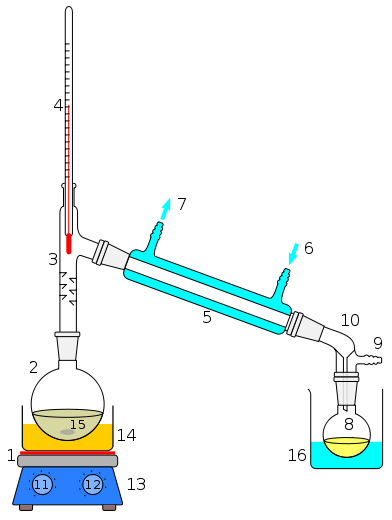
Figure 5:
Laboratory display of distillation: 1: A heating device 2: Still pot 3: Still
head 4: Thermometer/Boiling point temperature 5: Condenser 6: Cooling water in
7: Cooling water out 8: Distillate/receiving flask 9: Vacuum/gas inlet 10: Still
receiver 11: Heat control 12: Stirrer speed control 13: Stirrer/heat plate 14:
Heating (Oil/sand) bath 15: Stirring means e.g.(shown), anti-bumping granules or
mechanical stirrer 16: Cooling bath. (source:
wiki)