Phase Diagrams of Pure Substances
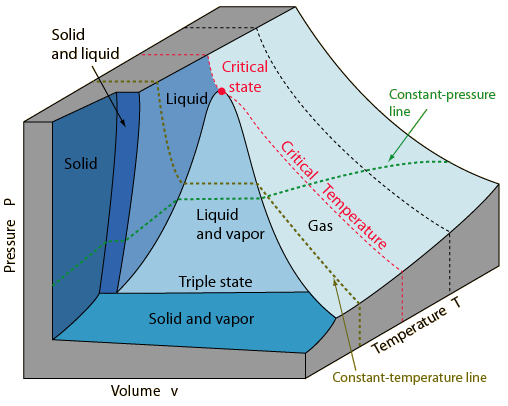
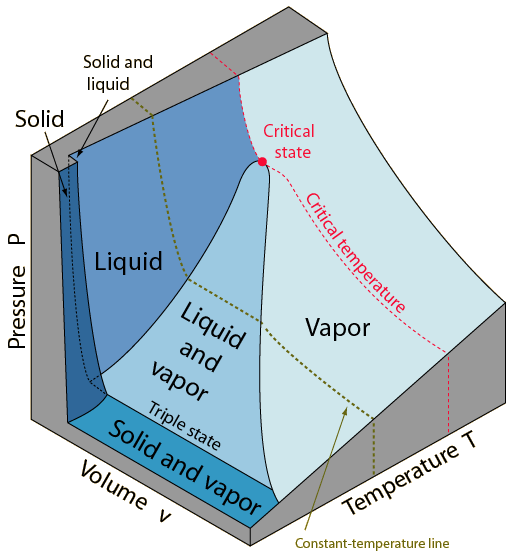
The
PvT
surface above represents a substance which contracts
upon freezing. Most
substances do so, the notable exception being water, bismuth and antimony which
expands upon freezing. The
expansion of water upon freezing has an enormous impact on the nature of the
Earth.
The PvT surface below represents a substance which expands upon freezing.
The solid, liquid and
gas (vapor) phases can be represented by regions on the surface. Note that there
are regions on the surface which represent a single phase, and regions which
are combinations of two phases. A point lying on a line between a single-phase
and a two-phase region represents a "saturation state". The line between
the liquid and the liquid-vapor regions is called the liquid-saturation line
and any point on that line represents a saturated-liquid state. A point on the
boundary between the vapor and the liquid-vapor regions is called a
saturated-vapor state. On these lines the phases are in equilibrium.
Note the critical state
where the saturated-liquid and saturated-vapor lines meet. The state variables
of this unique point are denoted by Pc, vc and Tc.
If a substance is above the critical temperature Tc, it cannot
condense into a liquid, no matter how high the pressure. This merging of the
liquid and vapor states above the critical temperature is a characteristic of
all known substances. While a pure vapor state can exist at a pressure lower
than Pc, at pressures above Pc it is constrained to be a
vapor. States with pressures above Pc are described as
"supercritical states".
The remarkable "triple
state" of matter where solid, liquid and vapor are in equilibrium may be characterized by a temperature called the
triple point. The triple state is represented by a line parallel to the Pv
plane with a characteristic pressure for the substance but variable volume. The triple point temperature of water is assigned the
value 273.16 K and the triple state of water is used as the reference for
establishing the
Kelvin
temperature scale.